|
ACO Quality Measures Performance Tool &
A New Tool for Assessing Depression in All Pateints 18 & Older
SETMA, through GTPA, is participating in a physician led Accountable Care Organization (ACO), which has already been approved by the Centers for Medicare and Medicaid Serivces (CMS). Lie the Medicare Advantage (MA) program with which we have been working for 16 years, the ACO has a set of quality metrics associated with it. SETMA’s deployment of a solution for the MA STARS Program can be reviewed at STARs Tutorial.
Unlike the MA STARS Program which has a graded performance from 3.0, which is the minimal acceptable, to a 5.0 which is the highest, the ACO quality metric set is an “all or nothing” requirement. If you do not reach the ACO Quality metric threshold, it is not possible to receive increased revenue no matter how much savings is created by the ACO. The following is SETMA’s design and deployment of a performance, tracking and auditing tool for the ACO quality measures set.
Legend
The ACO quality metrics can be reviewed at the ACO button on SETMA’s AAA Home. In addition, like all of our quality metrics, if the measures applies to the patient and has been completed for that year, the measure will appear in black; if the measure does not apply to the patient it will appear in grey; if the measure applies to the patient and has not been completed for the year if will appear in red.
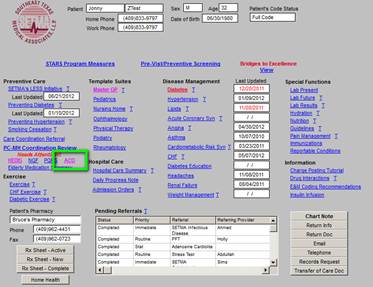
The ACO metric set is as follows. Each of he elements of this set will be review in turn. These are common quality metrics, all of which we have been tracking in other settings. The only new is “depression screening.” That will be discuss later. At AAA Home, when you deploy the button ACO, the following will appear. If you click the “view” button, it will show you what the content of each element is, which content is show below.
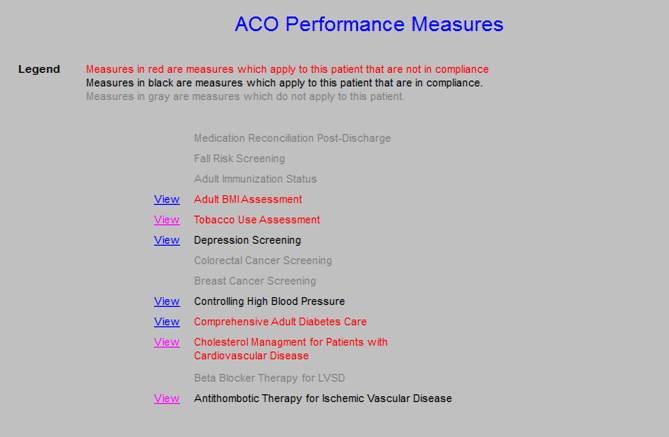
SETMA has measured Medication Reconciliation Post Hospital Discharge since we deployed the Physician Consortium for Performance Improvement (PCPI) in June, 2009, when it was first published. One thing will become apparent is how minimalistic the ACO Quality Metrics are. Medication errors account for more the half of the 30-day readmissions to the hospital. Yet, the ACO metric for Medication Reconciliation measures whether reconciliation was done within 30- days of discharge. SETMA is going to change that to three day. Over the past 40 months, we have discharged over 12,000 patients from the hospital. Regardless of age, we have completed medication reconciliation at the time of discharge 98.7% of the time.
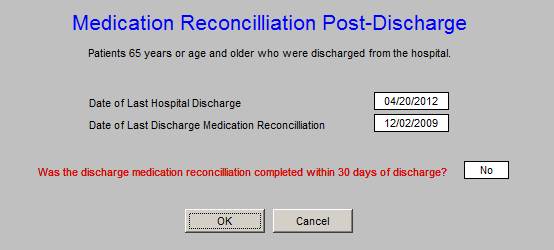
SETMA has been measure Fall Risk Assessment for more than five years. Through the National Quality Forums “Care for Older Adults,” SETMA has completed the Fall Risk 98.2% of the time as an organization. This function is already in our nursing and provider work flow.
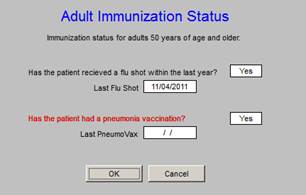
Adult Immunization status is a HEDIS metric for those who are over age 50. This is a metric on which SEMTA has been performing excellently for several years.
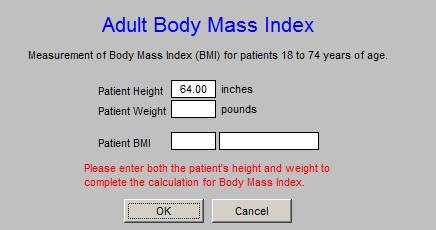
The Body Mass Index was added to HEDIS measures several years ago. Any adequate EMR which automatically calculated this value when the patient is weighed. SETMA weighs every patient every time they attend clinic and the BMI, the BMR and the Body Fat Percent are all calculated as part of the “weight management assessment” done on all patients.
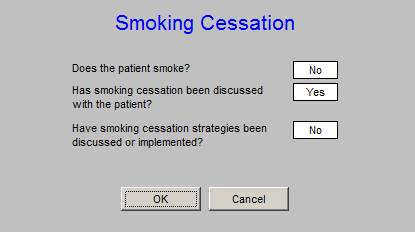
As part of the LESS Initiative (Lose Weight, Exercise, Stop Smoking) each patient is asked if they use any type of tobacco product. The effects of primary, secondary and tertiary smoke exposure is address with each patient. This metric is already being met by SETMA providers.
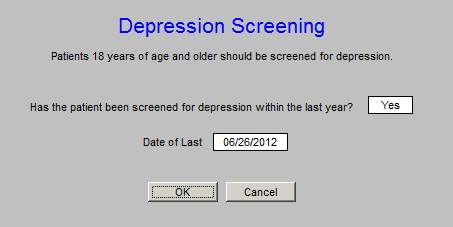
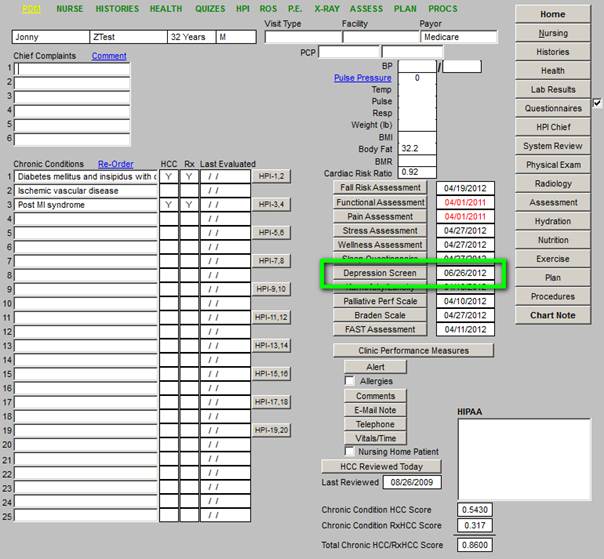
The Depression Screening is a new function for SETMA deployed for the purpose of fulfilling the ACO Quality metric requirement. The Depression Screening Tool will be discussed at the end of this presentation.
Multiple Quality Metric Sets include Colorectal Cancer Screening which reflects the fact that Colon Cancer can be cured if caught early enough.
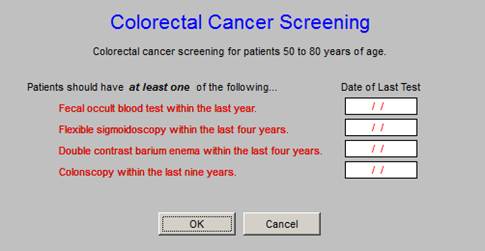
Breast Cancer Screening is a part of SETMA’s work flow. The age range is critical to this measure as it is from 40 years of age to 69 years of age.
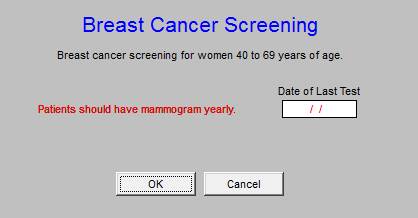
The discriminators for this metric make it different from other BP metrics. It addresses whether or not the blood pressure has been well controlled for he past three months. This means that depending upon the time of reporting, the patient may or may not be in compliance. This metric makes the repeating of the blood pressure during a visit very important if it is initially elevated.
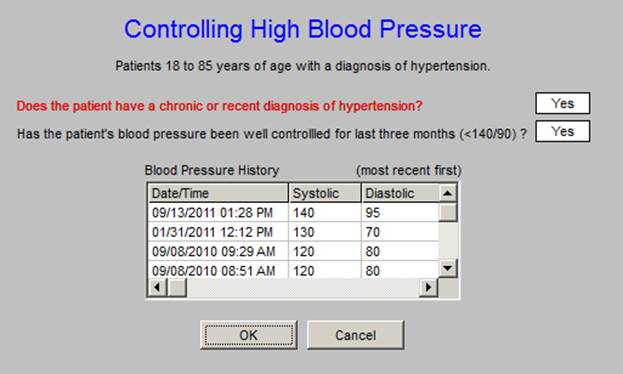
The Comprehensive Diabetes Measure is not new to SETMA. The ACO standard adds another Quality Metric Set to the existing nine measurement sets. This metric will be no problem for SETMA providers who are already measure each of these elements.

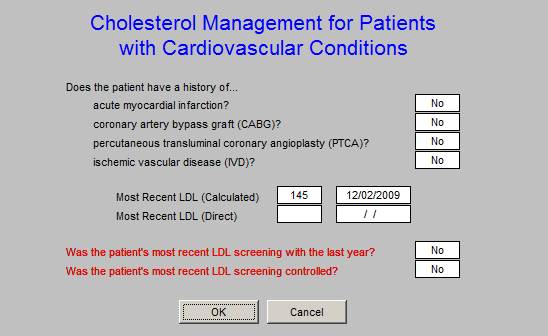
This Cholesterol Management Quality Metric Tool is different from others. It intends to identify those patients who objective are at high risk due to the present of one of four historical events which reflect the high risk status. It is this sub-population which this metric will address as to LDL testing and whether the patient is to goal. The “calculated” and/or “direct” LDL will be used for this measure.
This final ACO metric addresses Ischemic Vascular Disease. The use of aspirin or other antithrombotic fulfills this metric.

A Tool for Depression Screening of all patients from 18 and Older
The new Screening for Depression is found on the AAA Home template (see below outlined in Green). This screen should be completed once a year on all patients 18 and above. Like the the other screening tools, the first time it is completed it will take longer. Subsequently, if the information has not changed, it will only take one second to complete the tool.
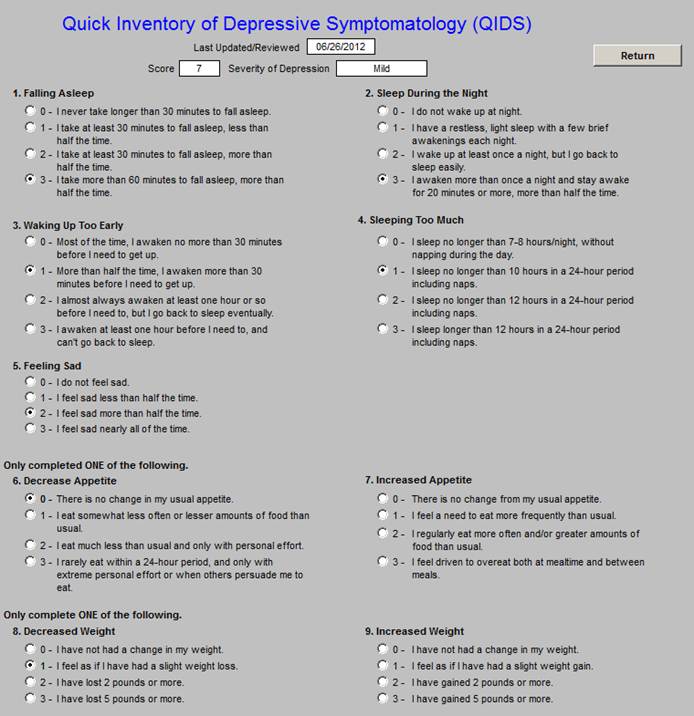
The content of the depression screening took is as follows:

A Summary Comment about the ACO Quality Measures
At the start, the final rules governing accountable care organizations emphasize quality measurements.
Provider groups planning to participate in the Medicare Shared Savings Program (MSSP) as accountable care organizations (ACOs) now know the lay of the land. Released by the Centers for Medicare & Medicaid Services (CMS) last October, the final rule outlines, among other things, the data reporting requirements ACOs will need to meet for inclusion in the plan.
A Focus on Quality
The final rule outlines 33 quality reporting measures, which were whittled down from the 65 included in the previous version. “They focus on clinical processes and patient experience-of-care measures,” says Shefali Mookencherry, MPH, MSMIS, RHIA, principal consultant of strategic and advisory services for Hayes Management Consulting.
The rule’s 33 measures are categorized into four domains: patient/caregiver experience, care coordination/patient safety, preventive health, and at-risk population care. Adequate reporting of the nearly three dozen measures will be the focus during the first year of the program. “Basically what happens is, for the first year, ACOs just have to report on these quality measures,” Mookencherry says, adding that in years two and three, ACOs will begin transitioning from a reporting-only to a pay-for-performance structure. By year three, all but one measure will become pay for performance.
“For the performance measurement, it’s all on the quality side,” says Ken Perez, director of healthcare policy and senior vice president of marketing at MedeAnalytics. Cost data, he explains, are already transmitted to the CMS through claims activity and other existing data submittal processes, so “there’s no reporting required on the cost side.”
In a MedeAnalytics white paper, Perez’s team says the rule’s approach is structured “to encourage participation and reduce the reporting burden,” and the criteria show “a clear preference for ambulatory-related measures.” Patient experience-of-care surveys, readmission figures, EHR use, and screening and immunization data are among the measures encompassed in the final rule’s first three domains, while the fourth, which covers at-risk populations, includes metrics on diabetes, hypertension, ischemic vascular disease, heart failure, and coronary artery disease. Several of the at-risk population measures-most of the diabetes metrics along with both measures related to coronary artery disease-are of the all-or-nothing variety.
How We Got Here
To reach the final rule’s 33 measures, experts say the CMS made great efforts to understand provider feedback and address concerns where possible. “CMS has been very clear that they heard what the public had to say in the public comment period before issuing the final rule,” Mookencherry says.
After careful evaluation of more than 1,300 comments, the rulemakers implemented a host of changes, including mitigating or removing much of the MSSP criteria surrounding meaningful use, although the incentive program itself remains unchanged. However, Mookencherry says some meaningful use criteria are still included. For example, EHR use is no longer a requirement, but the metric carries greater weight than other measures and will allow ACOs that use the technology to earn higher quality scores. This is likely to encourage ACOs to leverage EHRs in some form, according to Mookencherry.
Some of the public comments and CMS responses, which are summarized in the final rule, were quite conversational, leading many experts to believe the highly interactive process and the federal agency’s responsiveness resulted in enormous improvements to the draft regulations.
“The changes from the proposed regulations to the final regulations were extensive-and they were positive changes,” says Karen Ferguson, associate director of regulatory affairs at the American Medical Group Association (AMGA) who believes that because the program is voluntary, the CMS was keen to make the business case positive and strong.
“It really shows that CMS did pay attention to stakeholder input, and they really want the program to work,” Ferguson says. “They were very, very responsive to the public comments.”
Overlapping Data
Organizations already participating in federal programs will likely see significant overlaps between the data they are already reporting and the measurements required under the ACO/MSSP final rule (see table). Items such as EHR use tie into meaningful use, Mookencherry says, and are also part of the ACO measures. In addition, the clinical quality measures “are similar to the Physician Quality Reporting System [PQRS],” she says, which may be reported through the group practice reporting option, a Web-based data collection tool and the same portal ACOs will use to submit data on their 33 quality measures.
“You can see CMS is trying not to have duplicative systems out there where you have to enter things many times,” Mookencherry says.
“We’ve done an analysis of all 33 quality measures,” Perez says, “and we’ve found out that all but one overlap with an existing quality reporting system or program.” Two of the measures overlap with Agency for Healthcare Research and Quality indicators, 25 align with Healthcare Effectiveness Data and Information Set measures, seven overlap with meaningful use stage 1 measures, 13 overlap with PQRS measures, and one overlaps with the Hospital Value-Based Purchasing program. Because all but one of the ACO/MSSP measures are more or less known quantities, Perez says “there shouldn’t be much terminology shock, if you will, regarding the measures.”
John Cuddeback, MD, PhD, chief medical informatics officer at Anceta, AMGA’s collaborative data warehouse, says, “CMS has really tried to make the measures as consistent and as well aligned with other reporting programs as they could.”
Those measures that align with what’s already in the PQRS measure set “are very typical, very reasonable measures to reflect population health,” according to Cuddeback, adding that the ACO/MSSP measures are “good indicators for overall population health, with a focus on chronic disease and a focus on the high-cost, high-prevalence conditions that are responsible for the majority of the costs in Medicare.”
Pulling It All Together
Because of the large number of overlaps between the measures reported under the final rule and other federal programs, many ACOs won’t need to reinvent the wheel. However, there are some key infrastructure and process points that will facilitate gathering the necessary data sets.
“While the way these measures are reported can technically be done with paper charts,” Cuddeback says, “the people who have been doing this for a while find that an electronic health record is really important to be able to do this efficiently.” The mechanics of collecting data for submission sets the stage for a care process design that ensures patients are receiving good care while simultaneously monitoring favorable performance on the program’s measures, he adds.
“It really encourages organizations to think about planning the encounters and having support systems in place, both people and technology, to ensure good care coordination,” Cuddeback says.
In a report released in August 2011 prior to the publication of the CMS’ final rule, KLAS Research talked with organizations pursuing ACO and meaningful use initiatives about the trend toward greater reliance on business intelligence (BI) tools and methodologies. “They mentioned needing to invest in data warehousing and BI tools that will help them do this kind of reporting,” says Colin Buckley, the research firm’s strategic operations manager.
While reporting is critical for ACOs, many organizations identified a bigger picture concern of being able to fully analyze data internally to better understand what’s going on with patients. Without that knowledge, Buckley says, “They won’t be able to make good patient-by-patient decisions or do population disease management as well in order to understand what are the best policies for providing care.”
He believes ACOs are likely to dovetail the infrastructure needed to report under the final rule with that needed to mine the data for ways to improve patient care. He also says that while much of the information required under various initiatives is similar, it’s “not exactly the same, which causes a lot of consternation for folks as to why the government can’t measure the same things the same way for different programs.”
According to Mookencherry, another major point of concern will be identifying the gaps in communication streams, such as those between existing IT tools and caregivers. EHRs may or may not be tied into revenue cycle systems, record-keeping methodologies may be deeply entrenched, and other operational processes could need updating. It will be critical to ensure that everyone is on the same page, which may include things like transitioning physicians away from paper documentation and leveraging nursing input on the program’s quality measures. “There are some challenges there,” Mookencherry says, “but the key is really the communication, the use of IT, and the physician, nursing, and operational buy-in.”
Corrective Actions
For ACOs struggling to get their reporting processes in gear, experts say several communication channels will be available to help them conform to the program. The 10 CMS regional offices across the country may offer organizations a less intimidating avenue than contacting the main office in Washington, DC, as well provide a local resource that may be familiar with a particular area’s ACO.
“The final rule does talk about the opportunity to take corrective action,” Ferguson says. “It talks about CMS contacting the ACO with any concerns to give them the opportunity to get back on track.” Once those communications are under way, she says any failure to meet the minimum standards of performance will result in a formal warning, allowing an ACO the chance to “take corrective action before the end of the performance period.”
Smart ACOs will be prepared for audits down the road. “I know they’re planning on doing audits on the data that is submitted,” says Mookencherry, who adds that, among other parameters, ACOs should be prepared to reproduce reports to satisfy auditors as well as clearly demonstrate how various metrics were recorded and scored. Based on the type of issues an audit may uncover-data inconsistencies, subpar record keeping, etc-she says the CMS may choose to give an ACO a second or even a third chance. But if an ACO isn’t able to demonstrate solid compliance and reproducibility, Mookencherry says the CMS will “probably just toss them out of the ACO program.”
Perez believes the CMS wants ACOs to be successful within the program and says a grace period will allow time to evaluate and fix outstanding issues, be it unclear submissions or a lack of timely reporting. However, if the time frame passes without those problems being resolved, he says the final rule clearly spells out potential consequences: The noncompliant ACO will likely find itself out of the program.
“Let’s say you sign up for a three-year agreement for the Medicare Shared Savings Program,” Perez says, “and after one year you do OK but the second year you just ignore it and you don’t abide by the reporting requirements. CMS has the ability to terminate that ACO from the program immediately.”
What’s Next?
Even though the CMS incorporated a significant amount of feedback and suggestions when formulating October’s final rule, most experts agree the ACO reporting and performance requirements are likely to grow. “Certain things about it could evolve,” says Ferguson, who expects the CMS to continue reaching out to providers for ongoing discussions. “The other thing that needs to be considered is that the ACO program is just one of many federal initiatives,” she notes.
In her opinion, it’s likely that it and other programs will eventually be tied to full reimbursement under Medicare. She believes the totality of resources involved in organizing as an ACO, reaching out to partners as part of those efforts, preparing for ICD-10 compliance, and the other issues facing the healthcare industry have the potential to be “disruptive” and that participants should expect a learning curve.
One trend Buckley sees on the horizon is more technology vendors entering the healthcare arena. If ACO reporting requirements grow over time, that influx is likely to become more pronounced. He believes there’s been a shift toward enterprise-level vendors within the healthcare space and says ACOs are increasingly looking outside their core healthcare technology resources for tools to manage clinical and financial analytics. “That doesn’t necessarily mean completely outside the industry,” Buckley explains, “but what we’re seeing is vendors outside the industry coming in.”
“Anything that affects something as large and complex as the healthcare system as a whole is going to be disruptive,” Cuddeback says. “I think it’s important to recognize that people have an awful lot on their plates,” including ICD-10 projects and meaningful use efforts, as well as the changes coming in conjunction with PQRS requirements. “Beginning in 2015, providers won’t get full reimbursement unless they’re doing PQRS reporting,” he says.
Given the range of issues providers are dealing with, Cuddeback believes the ACO regulations have tried to be “as consistent and reasonable as possible” and commends the CMS team for what he says has been a thoughtful job of casting the regulations in ways that are as uniform and well aligned as possible “with everything the providers have to do and with the goals of the program.”
|