|
Genetics and SETMA’s Dr. Anwar
Several weeks ago, Dr. Anwar, SETMA’s Medical Director saw a 62 year-old lady with a history of hypertension, elevated cholesterol and rheumatoid arthritis. She had a strong family history of cerebrovascular disease. Dr. Anwar did a complete workup which included the usual evidenced-based tests. The patient was very concerns about “having a stroke.” Because of her concern Dr. Anwar seriously thought about giving her Plavix rather than just putting her on aspirin.
Because of her concern and with her permission, Dr. Anwar did a “cardiovascular DNA (Genetic) Panel.” It showed that she had significantly reduced levels of the CYP2C19*2/2 enzyme that is essential for the patient to produce the active metabolite of Plavix. Without that metabolite, Plavix cannot work. This makes Plavix a totally useless medicine in this patient. And, it is probable the reason why her father had repeated strokes even though he was treated with Plavix.
Even though Dr. Anwar was going to treat her according to the current established guidelines, it would not have protected her against strokes. Armed with this new information, Dr. Anwar changed her treatment to a higher dose aspirin therapy. This remarkable story foreshadows the future of healthcare which will be much more effective because of genetic testing.
Genetics
The complete sequencing of the human genome in 2003 has opened doors for new approaches to health promotion, maintenance, and treatment. Genetic research is now leading to a better understanding of the genetic components of common diseases, such as cancer, diabetes, and stroke, and creating new, gene-based technologies for screening, prevention, diagnosis, and treatment of both rare and common diseases.
However, this knowledge raises new ethical issues some of which are::
- Privacy and Confidentiality: Who should have access to genetic information? Who owns and controls it? How can families resolve conflicts when some members want to be tested for a genetic disorder and others do not?
- Discrimination: Should employers be able to require job applicants to take genetic tests as a condition of employment?
- Equitable Access to Genomic Technologies: Resource-poor nations, the uninsured, rural and inner city communities €“ How might genomic science and treatments be made available to those with fewer resources? Rare genetic conditions €“ Who will fund the development of treatments for genetic disorders that affect a relatively small number of people?
- Impact of Genetic Information: How does a person’s genetic information affect that individual and society’s perception of that individual? How does genetic and genomic information affect members of minority communities? (Adapted from Human Genome Project Information, 2007)
Genetics also introduces a vast amount of knowledge into the practice of medicine making it impossible for anyone to learn all of it. Therefore, technology, particularly electronic medical records are going to be required in order effectively and accurately to use genetics in daily medical practice. Some of the next steps of for key players in medical genomics are:
- Health-care providers. Learn to use electronic medical record (EMR) enabled tools and to apply genomic information to clinical decisions.
- Patients. Define preferences about what information they wish to learn and have placed in their EMR.
- Clinical molecular geneticists. Interpret how genetic results change biological function, including variants that warrant clinical action.
- Bioinformaticians and computing specialists. Develop curated genomic databases and means to query them to guide clinical decisions.
- Genetic counsellors. Provide information about the potential burden of results and implications for family members.
- Policy-makers. Define the rules to return results to patients and ensure privacy and confidentiality.
- Researchers. Develop consensus on how best to conduct research using EMRs linked to genomic information.
- Translational specialists. Train people across disciplines to communicate effectively and provide the 'glue' for the system to work.
- Software vendors. Design effective EMR and related systems.
In the face of new knowledge, SETMA has built a tool which allows SETMA providers to use genetics for the benefit of our patients. The following template displays how to order these tests through the EMR.
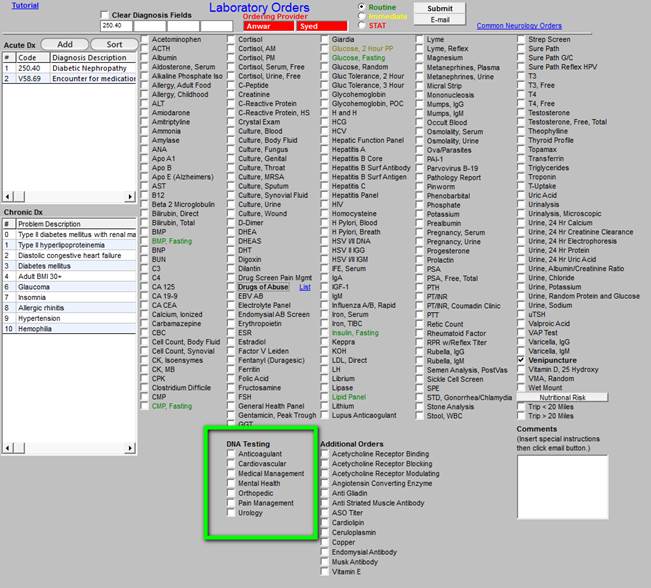
The box outlined in green allows providers to order genetic testing for the following conditions.

As more genetic data are incorporated into EMRs, health systems can carry out 'pragmatic clinical trials' - those that randomize groups of clinicians to study an intervention (in this case treatments guided by genetic information) compared with usual care. Although standards for how results should be assessed and adopted still need to be worked out, such studies are cheaper than conventional clinical trials and can collect data from people with multiple conditions, who are often excluded from trials.
To translate genetic analysis into improved medical care, hospital executives and other leaders must redesign how they collect and manage data so that it can be used in research. Existing patient databases for clinical care are often inadequate for evaluating health interventions: data are frequently missing or incorrect, and it can be hard to link data about, for example, demographics, appointments, procedures, medications and vital signs.
Pioneers of learning health-care systems have established streamlined consenting processes and data warehouses that link EMRs with genomic assays from patients' banked biospecimens. However, systems are not consistently incentivized to build these capacities. Health leaders want evidence before investing resources, yet without those investments, such evidence is hard to gather.
This makes the experiences from diverse health systems all the more precious. In January this year, a nascent organization of countries and international organizations met to discuss their efforts to evaluate how and whether genetic variants can improve clinical care. Programs are a blend of data collection (bringing genomic information and medical records together), clinical implementation (getting providers to use information) and outcomes (assessing the impact of using information).
But how will the use of genetic variants in medical decisions move from exploratory to mainstream? The US Centers for Disease Prevention and Control has developed a useful framework to evaluate new genomics tests. It includes whether a test measures genotype accurately, how reliably it provides information about a diagnosis or outcome, how likely it is to improve outcomes and whether the test has ethical, legal or social implications, such as uncovering false paternity or future untreatable conditions.
The medical-genomics community now needs to focus on how to decide what levels of evidence are required to demonstrate that assessing a variant helps patients. For example, a variant that may require a change in diet might need less evidence than one that may require an irreversible surgical procedure. The clinical community should commit to developing ways to evaluate genetic variants as 'actionable', including understanding how results from one patient population might apply to another with differing genetic backgrounds or health practices.
We are early in the use of genetics in routine medical practice but within the next ten years, it will become routine.
|